The Nonlactating Human Breast
Authors
INTRODUCTION
The evolution of the mammary gland from a sweat gland-like skin organ occurred almost 100 million years ago. This unique development was in a large part responsible for the rapid proliferation of the class of vertebrates that we now know as mammals. The advantage of the mammary gland was that it provided a ready source of nutrition for the still-developing infant, obviating the need for prolonged incubation in a fragile and vulnerable egg encasement. From a functional point of view, there are those who would contend that the human mammary gland has outlived its usefulness; however, the discovery of the multiple valuable and unique properties of human milk for humankind and the devastating results of attempting nonlactational nutrition for infants in many of the developing nations of the world would argue strongly against this point. We have just begun to appreciate the potential long-range sequelae of breast-feeding biologically and behaviorally.
EMBRYOLOGY
The long and complex evolutionary development of the mammary gland from a simple sweat gland can be seen within the mammalian family. The duckbilled platypus has only two simple tubules, which, under the influence of oxytocin, expel their contents onto the hair of the mother, from which it is licked away by the young. In the marsupial the beginnings of nipple formation can be observed. Further differentiation has resulted in the efficient organ of milk production seen in higher mammals.
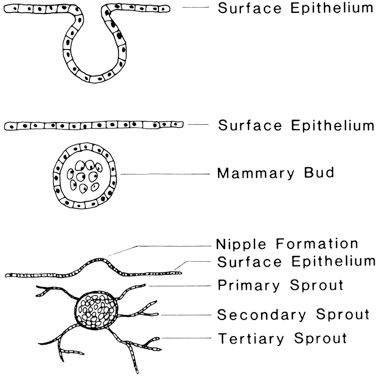
The first visible sign of mammary formation in the human embryo is a thickening of cells within the malpighian layer of the epithelium along the ventrolateral surface at approximately 35 days of development. This mammary ridge is seen primarily in the axial-thoracic area in the human being, but it can occur anywhere from the cervical region to the thighs. The area of prominence in the embryo corresponds to the area of future mammary development in the adult.
The mammary ridge is present for only a few days during development, after which specific paired areas of prominence begin to develop in the locations characteristic for the species. These pairs of protuberance, called mammary buds, are evident in the 50-day-old embryo and are formed by the coalescence of specialized surface epithelial cells that invaginate into the underlying mesenchyme. Rapid cellular division occurs, giving rise to the formation of a sphere of cells beneath the epithelial surface that represents the actual mammary bud (Fig. 1). All subsequent mammary development depends on this bud of epithelial cells. One bud forms for each nipple; in the human being, two mammary buds form in the thoracic region.
|
By 84 days of development, epithelial outgrowths of the mammary buds can be seen invading the surrounding mesenchyme. The number of these outgrowths, or primary mammary sprouts, determines the number of openings per nipple (galactophores) that will be found in the mature gland. This number is also species specific, with 15–25 openings per nipple in the human being and only one in the cow. As these sprouts continue to develop and invade the mesenchyme, increased pressure under the surface epithelium gives rise to a protuberance of the surface that will further differentiate into a discrete nipple or teat. The human nipple usually develops as a proliferative-type protruding nipple; certain other mammals have an ingrown-type nipple that protrudes only in late pregnancy or with the stimulation of nursing. Canalization of the primary sprouts results in formation of the glandular and nipple cisternae at the proximal and distal ends, respectively, of each sprout. Branching of the primary sprouts, giving rise to secondary sprouts, begins at approximately the same time as canalization in the primary sprouts. The secondary sprouts branch once again to form tertiary sprouts, resulting in the formation of primary and secondary milk ducts, respectively (Table 1).1
Table 1. Stages of development of the human mammary gland
Mammary Development | Age of Embryo (days) | Length of Embryo (mm) |
Mammary ridge | 35 | 6 |
Mammary bud | 49 | 20 |
Nipple formation | 50 | 22 |
Primary sprout | 84 | 68 |
Secondary sprouts | 100 | 110 |
Myoepithelial cells | 140 | 160 |
Canalization of primary sprouts | 150 | 170 |
Milk ducts | 260 | 320 |
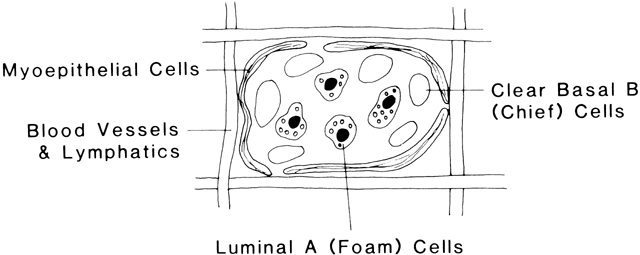
Another cell type having its origin from the same epithelium as the mammary bud is the myoepithelial cell. This cell is formed from an undifferentiated epithelial cell, and once formed it does not divide again. These cells are evident in the human being by 140 days of development, and a number of them surround each alveolus. Even in the embryonic form these cells contract on exposure to oxytocin, squeezing the alveolus into a tight sphere. The functional unit of the mammary gland is composed of the alveolus surrounded by myoepithelial cells drained by a tertiary milk duct (Fig. 2). The primary mesenchymal cell type composing the matrix in which the mammary gland develops is the fat cell. A substantial fatty pad is necessary for the extensive invasion by the mammary ducts during development of these structures during puberty and pregnancy. Adipose tissue known to be metabolically active may also provide local growth-promoting factors necessary for the full differentiation of the potential mammary-producing epithelial cells. Branching is also coordinated by local cross-talk between developing duct epithelium and nearby stromal cells. Tissue transplantation studies demonstrate that mesenchymal cues control the branching pattern of the epithelium, regardless of epithelial origin.2 The mesenchymal tissue also gives rise to vascular elements, which along with the neural tissue derived from the neural ectoderm complete the structures necessary for the future functioning mammary gland. This prenatal development of the mammary epithelial sprout serves as a critical event for further mammary gland growth. The mammary epithelial bud receives signalsfrom the surrounding fat pad to form primary ducts and beginextension into the fat before birth. Interference with this occurrence could lead to altered timing of mammary development or formation of the glandular structures (altered number of primary ducts or blind ducts or unusual presence of nipple/areola), leaving lasting effects on the gland.3
Mammary development in the male and female is essentially the same at birth. In some other species (e.g. the horse), no nipple formation occurs in the male due to a separation of the mammary bud from its connection with the surface epithelium early in development. This separation and failure of nipple development occurs as the result of proteolytic enzyme action initiated by testosterone. Exposure of the female to testosterone during nipple differentiation results in failure of normal mammary development. Conversely, exposure of the male to the antiandrogen, cyproterone acetate, results in formation of a complete mammary gland.4, 5 Exposure of the developing male embryo to testosterone apparently desensitizes the mammary bud to the eventual stimulation of rising estrogen levels. This is not an all-or-none effect, however, because the presence of gynecomastia in the human being is well documented.6
Clinical Correlations of Embryologic Development
Accessory nipples (polythelia) or mammary glands (polymastia) may develop anywhere from the knee to the neck. Such development occurs along the primitive milk ridge and results from either the differentiation of a mammary bud in a location not characteristic of the species or the migration of epithelial elements along the milk line to other locations. Polythelia occurs in approximately 1% of the population, with the location being predominantly above the normal midthoracic region in Japanese and below this area in Europeans. A familial occurrence of polymastia has been described, with accessory mammary glands in the axilla in several generations of the same family.7 Once recognized, polythelia and polymastia require no therapy other than patient education and reassurance. Occasionally such development requires surgical removal for cosmetic reasons.
The normally developed mammary gland of a newborn infant usually contains prominent glandular tissue under the nipple from which a milky discharge can be expressed. The mammary prominence and the nipple secretions result from the actions of fetal prolactin and maternal and placental estrogen on the responsive mammary tissue in utero. These changes diminish after birth and are usually absent by four months of age. Palpable mammary tissue does not occur normally again until pubertal exposure to estrogen occurs.
Another clinical entity, the pseudoinverted nipple, reflects an embryologic development appropriate to certain species of mammals other than the human being. This developmental abnormality results in a retracted but nonfixed nipple. Stimulation of the nipple causes it to become erect and everted.
POSTNATAL DEVELOPMENT
Pubertal Events
Hormonal Effects
The increase in hypothalamic-pituitary activity resulting in an increase in gonadal estrogen production is first seen as increasing size of the breast bud or thelarche. This earliest sign of secondary sexual maturation at the onset of puberty can normally occur anywhere from age 8 to 14 years. The process of mammary maturation that begins with puberty results in the development of a mature lobular-ductal-alveolar system over a span of approximately four years. The minimum hormonal requirements necessary for development of the mature nonlactating gland include estrogen, progesterone, prolactin, growth hormone, and insulin. Thyroid hormone, parathyroid hormone, and cortisol are probably facilitative as well. Data derived from tissue culture studies of mammary development are complicated by the inherent problem of requirements for tissue culture medium not necessarily needed in the in vivo situation. An additional complicating factor has been that many of the studies of mammary development have been in rodents.
The specific hormonal requirements for normal lobular-ductal-alveolar development in the human are largely unknown; however experimental evidence suggests that embryonic, adolescent, and adult phases of mammary development are differentially regulated. Adolescent branching, for instance, requires growth hormone (GH), estrogen, and estrogen receptor α (ERα); adult tertiary side-branching, on the other hand, requires progesterone and its receptor (PR); and embryonic branching apparently proceeds without any hormonal requirement at all, as it occurs normally in the absence of either ERα, ERβ, PR, or the receptors for GH or prolactin.2 For example, sexually immature rhesus monkeys given physiologic doses of estradiol achieve full breast development within two months. If the animals are hypophysectomized before estradiol treatment, the breast does not mature. These studies, completed by Kleinberg and coworkers,8 indicate that a functioning pituitary gland and estradiol are necessary for breast maturation. When hypophysectomized animals were given estradiol and growth hormone, breast growth occurred but was incomplete. To define the role of prolactin in breast development, intact animals were given pergolide to selectively suppress pituitary prolactin output. When these animals were given estradiol, normal breast development resulted. The investigators concluded that, in primates, estradiol, growth hormone, and other pituitary hormones are required for normal pubertal breast maturation.8 Prolactin may not be required during the actual period of growth and development, but exposure of the mammary gland to prolactin before estradiol stimulation still is considered essential.9 The mechanism through which prolactin influences breast growth may involve the induction of estrogen receptors by prolactin in breast tissue.10
During development, different tissues within the breast are responsive to different endocrine stimuli. The accumulation of adipose tissue appears to be estrogen dependent. In a facultative environment, estrogen stimulates extensive aborization and differentiation of the ductal epithelium. Completion of the lobular-ductal-alveolar configuration with budding of the terminal ductules and differentiation of the alveolar epithelium requires the synergistic effects of estrogen and progesterone on the glandular anlage.11
Adipose Tissue
Just as adipose tissue seems to be critical for normal embryologic differentiation of the mammary gland, it also plays an integral role in further development at the time of puberty. The deposition of adequate adipose tissue under the influence of ovarian estrogen is necessary to form the loose matrix in which the extensive glandular and ductal proliferation of puberty occurs. In tissue culture, mammary epithelial cells do not undergo mitotic division except in the presence of diffusible substances from mammary adipose stromal tissue.12 The local steroidogenic hormonal milieu provided by the adipose tissue surrounding the glandular epithelium undoubtedly creates a unique environment facilitative for mammary epithelial proliferation and differentiation. It may be that the mammary growth seen after oophorectomy in the presence of high doses of prolactin occurs because of local estrogen production by the adipose tissue from nonestrogenic precursors.
Clinical Correlations of Pubertal Development
Premature thelarche is defined as the growth of breast tissue before the age of eight years in the absence of other secondary sexual characteristics. This is a relatively common disorder that occurs before the age of two years in 85% of cases, with approximately one third of these cases present at birth. Premature thelarche that occurs before two years of age spontaneously resolves in most cases, a process that may take up to two years. If breast growth begins after the age of two years, the condition usually persists until puberty, when other sexual characteristics appear at the normal age. In any case of premature thelarche, a thorough endocrinologic evaluation is necessary to rule out an estrogen-secreting tumor, ingestion of estrogenic substances, and true isosexual precocious puberty.13
Girls with premature thelarche have a derangement in the maturation of the hypothalamic-pituitary-gonadal axis resulting in increased serum levels of follicle-stimulating hormone (FSH) and an exaggerated response of FSH to infusion of gonadotropin-releasing hormone (GnRH). Serum luteinizing hormone (LH) concentration and the LH response to GnRH are normal in these children. The increase in FSH stimulates ovarian estradiol production sufficiently to promote breast growth.14 In girls with true isosexual precocity, pulses of gonadotropin with LH dominant can be detected with frequent blood sampling; in girls with premature thelarche, the same techniques identify only pulses of FSH. This difference not only confirms the etiology of premature thelarche but provides a method to differentiate the two disorders.15 Studies suggest that up to 14% of girls with presumed premature thelarche progress to true precocious puberty.16 For this reason, patients with the diagnosis of premature thelarche should be monitored closely.
Unilaterlal or bilateral adolescent mammary hyperplasia can be a distressing situation to both the child and her parents. Excessive growth of primarily fat and connective tissue causes both psychosocial maladjustment and physical symptoms of pain, and shoulder and back strain. There has been little success in the medical arrest of this condition. In general, therapy is delayed as long as possible until the accelerated phase of mammary development is complete, and then reduction mammoplasty is the treatment of choice. Surgical therapy done prematurely usually results in recurrence of the hypertrophy. If symptoms demand early surgical reduction, ovarian suppression with leuprolide acetate or danazol for 1–2 years after the procedure should be seriously considered. A similar rare form of mammary hyperplasia occurs during early pregnancy.
Asymmetric mammary development, especially during early puberty, is the rule rather than the exception. Usually one mammary bud begins to enlarge before any noticeable enlargement has occurred on the other side. Many parents and some physicians have mistaken this unilateral enlargement for a tumorous mass. This error in diagnosis is particularly common in children with premature puberty, when the young age of the patient misleads the examining physician. Surgical excision of the small mammary bud at this stage of development results in total failure of breast development on the operated side. This tragic mistake still occurs far too often.
Athelia (failure of nipple development) and amastia (failure of mammary gland development) occur rarely in the human being. However, unilateral failure of breast development during puberty or extreme asymmetry is not uncommon. Failure of development of the mammary gland on one side may result from either abnormal development of the mammary bud during embryologic life or traumatic or surgical destruction of the mammary bud. There is no known hormonal therapy for partial or complete unilateral amastia. The clinical approach to the pubescent young woman with this condition should be patient support, education, and properly augmented undergarments until such time as pubertal development is complete. By age 18 years, or sooner in some individuals, mammary development is complete, and augmentation mammoplasty on the affected side should be considered if significant asymmetry persists. Some asymmetry in mature breast size and shape is usual. The important point is that it is impossible to determine the final degree of asymmetry until development has been completed. In some cases of asymmetry, glandular aplasia is present in the hypoplastic breast. Difficulty with lactation has been reported in these women.16
As suggested previously, mammary development does occur to some extent in the genetic male despite exposure of the mammary tissue to testosterone prenatally. Some degree of mammary development or gynecomastia is extremely common in the normal male at the time of puberty. This most likely occurs because of an increased ratio of estradiol to testosterone, which frequently exists during the maturation of the testicle. Palpable breast tissue beneath the nipples is present for several years but then regresses as testosterone becomes the dominant sex steroid.
The gynecologist or reproductive endocrinologist is occasionally consulted to evaluate a young boy with gynecomastia because of concern about a possible intersex disorder. A review of the causes of gynecomastia is, therefore, appropriate. Gynecomastia related to intersex disorders is almost always associated with ambiguous genitalia. The only genetic abnormalities of consequence with apparently normal male external genitalia are Klinefelter's syndrome (XXY) and the mildest of the incomplete testicular feminization syndromes. There is an idiopathic form of gynecomastia that differs from the other forms in that it produces full ductal and glandular development. Gynecomastia in other clinical settings is characterized histologically by fatty and fibrous tissue proliferation.
The other major causes of gynecomastia are organic disease and drug ingestion. Nontumorous (orchitis, atrophy) and tumorous (choriocarcinoma) lesions of the testicles may result in gynecomastia. Carcinoma of the adrenal cortex, certain pituitary adenomas, hyperthyroidism, liver disease, and starvation has been associated with the development of gynecomastia. Finally, exogenous medications, including both estrogens and androgens, digitalis, isoniazid, and spironolactone, have been associated with gynecomastia.
THE MATURE FEMALE BREAST
During the course of puberty the mammary gland enlarges and changes shape, undergoing the classic changes described by Tanner from simple nipple protuberance to the mature, nonlactational, dome-shaped mammary gland with a centrally located areolar mamma. Great variability occurs in the size, shape, and location of the mature mammary gland due to constitutional factors, differences in amount and location of fat distribution, connective tissue development, thoracic cage shape, and overall body height. For example, the taller the individual, the higher the breasts will be located on the thoracic cage. The overlying skin of the mature breast is fairly thin, and the dilated mammary veins are frequently observed. On the periphery of the areolae are located the small duct openings of the sebaceous glands of Montgomery. These glands contain no terminal hairs but do contain lanugo hairs. The areolae also contain smooth muscle fibers that contract on stimulation, wrinkling the skin of the areolae and causing nipple erection.
The majority of the glandular tissue of the breast is located in the central upper and outer quadrants of the breast. Not surprisingly, it is in these same locations that carcinoma of the breast most commonly occurs. In addition, there is a small tongue of glandular tissue, the axillary tail of Spence, that penetrates the fascia of the axilla and lies in close proximity to the axillary lymph nodes. This tissue is most prominent in the lactational state and is occasionally mistaken for lymphatic pathology.
Growth and development of the mammary glands continues after puberty, but at a much slower pace, during the cyclic fluctuations of the follicular and luteal phases of the ovulatory cycle. Rising estrogen concentrations during the follicular phase give rise to proliferation of the end-ductular structures, maximal at the time of ovulation. After ovulation, the combined effects of estrogen and rising progesterone stimulate further development and differentiation, particularly of the alveolar structures. As estrogen and progesterone begin to diminish toward the end of the cycle, prolactin-induced secretory changes become apparent in the alveolar epithelium, with secretory products appearing in the alveolar lumen during the first few days of the menses. The breasts reach maximum volume just before the onset of menses due to increased intracellular and intercellular water, maximal glandular and ductal development, and the accumulation of secretory materials within the alveolar structures. The breasts are smallest in volume and least stimulated on days four through seven of the cycle. The ideal time for examination of the breasts is during this period of minimal hormonal stimulation.
Many irregularities and small, cystically dilated glands appear during the latter portions of the cycle, under maximal hormonal stimulation, which are not palpable during the early portions of the new menstrual cycle. Similarly, symptoms of breast discomfort that are physiologic in origin rather than pathologic occur on a cyclic basis, being maximal during the last week of the cycle and minimal during the first half of the new cycle. A small quantity of nipple secretions also may be detectable on a physiologic basis during the last few days of the cycle and first few days of the new cycle.
The breast can no longer be considered a passive endocrine target organ. Recent information documents the capability of breast tissue to alter significantly the local hormonal environment. Aromatase and other enzymes necessary for the production and metabolism of steroids are present in the adipose tissue and glandular epithelium of the mammary gland. Circulating androgens can be converted locally to other, more potent androgens or to estrogens, modifying peripheral endocrine signals.17
A second mechanism through which breast tissue alters the local hormonal milieu is regulation of hormone concentration in ductal fluid. Analyses of ductal secretions have found high concentrations of estradiol and progesterone, much in excess of serum concentrations. In addition, prolactin and other proteins are present in this fluid. For most of these compounds there is little correlation between the concentration in serum and that within the ducts.18 The ability of the breast to modulate endocrine signals by conversion or concentration of circulating hormones explains the poor correlation between peripheral levels of hormones and pathologic conditions thought to be endocrine dependent. Understanding of the pathologic conditions of the breast and the selection of appropriate therapy will be enhanced greatly as the biochemistry and physiology of the mammary gland are clarified.
Sex Hormones and Breast Disease: Relationship and Therapy
Great controversy has existed concerning the possible relationship to the ingestion of sex hormones of both benign and malignant diseases of the breast. There is, however, a critical distinction that must be made between premenopausal and postmenopausal use and the risk of breast disease. Although there are reports to the contrary, the preponderance of existing data suggests no significant relationship between premenopausal exogenous hormone ingestion and benign or malignant neoplasia of the breast. Many studies, including the Cancer and Hormone Study, have demonstrated no association between breast cancer and the use of oral contraceptives.19 However, there are limited studies suggesting a small increase in breast cancer among young women with a positive family history. Most research has been done with higher-dose oral contraceptives, which are in limited use in the United States today. Although epidemiological studies have documented a small increased risk of breast cancer associated with older oral contraceptive formulations, recent studies that include newer formulations have not detected an increased risk.20
There is no association between breast cancer and the use of depot medroxyprogesterone acetate (DMPA), according to data from the World Health Organization.21 Concerns about DMPA and breast cancer are related to malignancies in beagle dogs, but this appears to be a species-specific phenomenon.
Fueled by the results of the Women’s Health Initiative (WHI), arguably the most controversial area is the potential effect of hormone replacement therapy (HRT) on breast cancer risk. Previously, the most comprehensive article on hormone therapy and breast cancer was published in 1997 by the Collaborative Group on Hormonal Factors in Breast Cancer, which analyzed 52,705 women with breast cancer and 108,411 women without breast cancer from 51 studies in 21 countries. The relative risk was 1.35 (95% CI 1.21-1.49) for women who had used hormone therapy for five years or more.22, 23 These results were similar to those of the Nurses' Health Study, which suggested an increased risk of 1.4 with long-term use, with no protective effect of progestins.24 In contrast, numerous studies have shown no effect, or even a benefit, with low-dose hormone use after menopause; however, most are limited by study design. The WHI, a long-term, prospective, randomized, placebo-controlled trial initiated by the National Institutes of Health, evaluated the risk of postmenopausal hormone replacement therapy. The results of the two study arms, published in JAMA in 2002 and 2004 respectively, demonstrated a small increase in breast cancer incidence in women taking combined conjugated equine estrogens with medroxyprogesterone (RR 1.24, 95% CI 1.03-1.66), whereas hysterectomized women who received conjugated equine estrogens alone had a slight reduction in their overall risk (0.77, 95% CI 0.59-1.01).25, 26 It should be noted, however, that there were limitations to the study design and no increase in breast cancer fatalities was reported by the WHI. Nevertheless, the small increase in breast cancer should always be considered prior to initiating HRT.
A similar controversy exists regarding the relationship between endogenous levels of sex steroids and the origin and pathology of fibrocystic breast disease (benign mastopathy). Histologically, this condition is defined by dense fibrosis in the stroma, epithelial proliferation with apocrine metaplasia, cyst formation, and alveolar atrophy.27 These changes are best explained by a relative or absolute estrogen dominance over progesterone. Hyperestrogenism frequently exists during the perimenopausal years or with chronic oligoanovulation, as seen in clinical settings where an increased incidence of fibrocystic breast changes has been observed. Because of the breasts' ability to modulate peripheral endocrine signals, levels of estrogen within ductal secretions may dominate even with seemingly normal ovarian sex steroid production.28 Fibrocystic changes are common; at least one third of women have breast cysts and epithelial hyperplasia found at autopsy.
Atypical lesions—atypical epithelial hyperplasia, atypical lobular hyperplasia, and diffuse papillomatosis with atypia—are associated with a higher risk for development of breast cancer. There may be as much as a doubling of breast cancer risk when atypical lesions are combined with a positive family history.29
The most common symptoms of fibrocystic breast disease include cyclic breast pain (mastodynia), tenderness, and palpable thickening with discrete cysts. A characteristic green nipple discharge is occasionally the presenting complaint. Often reassurance is adequate and no therapy is required for women with mild to moderate mastodynia. Nonhormonal treatments, including use of a supportive brassiere, a decrease in methylxanthines, and vitamin A and E use, have been suggested. Combination oral contraceptive pills are effective in alleviating symptoms in many women.30 Bromocriptine has been found to be effective in reducing mastodynia in double-blind trials,31 although side effects may make this therapy unacceptable.
Various synthetic progestins, given during the second half of the menstrual cycle, have been found to be effective in alleviating the symptoms of breast pain and engorgement.32 Clinical trials using danazol, administered in doses of 200–400 mg/day, have resulted in both subjective and objective improvements in fibrocystic breast disease after 2–6 months of therapy. Improvement was noted in approximately 60% of cases, but significant side effects were encountered in one third of the patients. Tamoxifen, as a partial estrogen antagonist, may be useful in the treatment of painful fibrocystic changes. At a dose of 10 mg/day, symptoms improve slowly over 2–3 months in two thirds of cases.33 The decision as to when to initiate therapy for fibrocystic breast disease and the selection of an appropriate therapeutic agent must be individualized, because the role of medical therapy in this disorder is not completely defined.
Nipple Discharge
Nipple discharge, found by the patient or by the physician during a physical examination, is one of the more common abnormalities encountered in the breast. The nature of the discharge is of great help in determining the appropriate evaluation and treatment. As indicated earlier, a slight amount of nipple discharge may be physiologic at the end of the menstrual cycle or during the first few days of the menses. Galactorrhea, which is the presence of physiologic milk secretion under nonphysiologic conditions, appears to be increasing in frequency. Whether this is occurring because of an increase in the exposure of individuals to substances stimulating mammary glandular epithelium, or whether an increasing awareness on the part of physicians of the importance of galactorrhea in relation to possible pituitary adenomas has resulted in more careful breast examination, is unknown at the present time. Galactorrhea is a milky nipple discharge that is present either spontaneously or on compression of the breast tissue and is confirmed by the finding of fat globules on staining of the wet preparation of oil red O or other fat-staining dyes. Galactorrhea does not indicate underlying inherent organic breast disease. Galactorrhea may be caused by medications that block the dopamine receptor, such as tricyclic antidepressants and antipsychotic medications. The antihypertensives reserpine and methyldopa may also cause galactorrhea. Rarely, galactorrhea has been reported with the use of higher-dose oral contraceptives.
Unexplained galactorrhea can be evaluated by measurement of the fasting prolactin concentration and the thyroid-stimulating hormone concentration to rule out an endocrinopathy. Prolactin-secreting pituitary adenomas and hypothyroidism can lead to galactorrhea. Commonly these conditions are also associated with amenorrhea.
Intraductal papillomas often manifest with a serosanguineous or bloody breast discharge. Mammography or ultrasound examination may reveal a dilated duct near the nipple. Surgical excision of the papillomas is recommended, because cytology cannot definitively diagnose a benign papilloma.
The presence of a sticky or multicolored nipple discharge usually occurs in the perimenopausal age group and reflects the presence of duct ectasia. The discharge is frequently associated with a burning and itching sensation of the nipple. If a mass is palpable, it should be biopsied.
A purulent nipple discharge is almost always associated with an infective process such as acute puerperal mastitis, chronic lactation mastitis, or simple abscess. Antibiotic therapy for a diffuse infection and occasionally surgical drainage for organized infection are the treatment choices.
Watery, serous, serosanguineous, and bloody nipple discharges should be considered symptoms of underlying carcinoma of the mammary gland until proved otherwise. In patients older than 50 years of age, carcinoma of the mammary gland is the leading cause of discharge of these types. Cytologic analysis of the discharge should be obtained whenever possible. If the slide is carefully prepared, one should expect a positive cytologic finding in 80% of the cases in the presence of carcinoma. In the younger age group, benign cytology findings, a normal breast examination, and normal mammography are usually sufficient to evaluate these forms of nipple discharge.
Breast Mass
The most common presentation for breast cancer is a breast lump. In women older than 50 years of age, 23% of breast masses are breast cancer. In younger women, most breast masses are benign, with only 4% representing cancer.34 Risk prediction models can be used to estimate the risk of developing breast cancer and predict the likelihood of carrying a BRCA1 or BRCA2 gene mutation. All risk assessment models have strengths and limitations, and no model is comprehensive enough to stand alone as predictive of breast cancer risk.35 Because the majority of women with a breast mass are first seen by their obstetrician-gynecologist, it is critical for these physicians to be well trained in the evaluation of breast masses (Table 2).
Table 2. Factors associated with an increased risk of developing breast carcinoma
Increased-risk patients
Late first pregnancy
Nulliparous
No lactation
Family history of breast and other malignancy
Previous breast surgery
Extreme-risk patients
Mother and sister with breast carcinoma
Mother or sister with bilateral breast carcinoma
Mother or sister with early (<35) onset breast carcinoma
Breast self-examination (BSE) for detection of masses is somewhat controversial. Although studies suggest that BSE can detect cancer at an earlier stage, before nodal involvement, other research suggests that BSE does not significantly improve the rate of early detection of breast cancer. Still, most health organizations and practitioners recommend monthly examinations. The examination should include an evaluation of the skin for retraction, dimpling, or inflammatory changes. Palpation of the axillary area is also important to include with the breast examination.
The American Cancer Society recommends that a clinical breast examination (CBE) be part of a periodic health exam, preferably at least every three years for women in their 20s and 30s then annually thereafter. Women who choose to do BSE should receive instruction and have their technique reviewed on the occasion of a periodic health exam. It is acceptable for women to choose not to do BSE, or to do BSE irregularly.36
In addition to breast self-examination and physical examination by a trained examiner, mammography plays an integral role in the early detection of breast cancer. Several large studies have documented the effectiveness of mammography as a screening tool that increases the detection of stages I and II cancers. However, despite the acceptance of this modality, the precise screening interval remains somewhat controversial. Nearly all North American organizations recommend initiating screening mammography starting at age 40. An earlier examination is warranted when there is a personal history of breast cancer or a history of premenopausal breast cancer in the patient's sister or mother. Mammograms should be obtained every 1–2 years from age 40–49 years, the precise frequency depending on the patient's family history and the ability to perform a satisfactory physical examination. After 50 years of age, annual mammograms are recommended.37
For patients at higher risk, screening MRI is recommended for women with an approximately 20–25% or greater lifetime risk of breast cancer, including women with a strong family history of breast or ovarian cancer and women who were treated for Hodgkin’s disease.38
Mammography also is important in the evaluation of a palpable breast mass as an adjuvant to fine-needle aspiration. With a documented malignancy, mammography helps to define the required extent of the resection and to determine the presence or absence of a subclinical lesion in the contra lateral breast. This modality is also very helpful in ruling out significant pathology when breast pain or nipple discharge occurs without a palpable abnormality. Finally, a mammogram should be obtained when a woman has detected an abnormality on breast self-examination that cannot be confirmed by a trained examiner. The properly obtained and evaluated mammogram should yield an accuracy of approximately 90% in the presence of carcinoma. Only one in 40 palpable breast masses harboring a neoplasm show no abnormality on mammography.
Once a breast mass is detected by examination and/or mammography, further evaluation can include ultrasound, fine-needle biopsy, core biopsy, or excisional biopsy. Ultrasound is useful in differentiating cystic masses and fibroadenomas from more concerning lesions. A simple cystic mass identified by ultrasound typically requires no further therapy. Aspiration can be performed if the cyst causes discomfort or concern. Fibroadenomas have clear margins by ultrasound and have low-level homogenous internal echoes. Fine-needle aspiration or excision can be performed when there is concern about the lesion based on the patient's history and age.
Solitary, discrete lesions can also represent intraductal papillomas or, rarely, lymphomas. The presence of multiple large masses suggests phyllodes tumors. Because phyllodes tumors can be malignant, complete excision is recommended.
Suspicious lesions on mammogram and ultrasound are most typically seen as irregular masses with spiculated or indistinct borders. Calcifications are often seen within these masses. Benign lesions, such as sclerosing adenomas, radial scar, and fat necrosis, can mimic these findings. Mammography can also be used for needle localization of masses before biopsy or excision.39
Breast cancer care requires a multidisciplinary approach. The team of breast experts includes primary care physicians, geneticists, breast radiologists, breast pathologists, surgical breast specialists, and radiation and medical oncology specialists. The complexity of breast cancer has increased with the advent of risk-reducing strategies, breast imaging technology, new drug therapies, and the integration of genomics into the evaluation and treatment of patients.35
REFERENCES
Gasser RF: Atlas of Human Embryos. Hagerstown, Harper & Row, 1975 |
|
Sternlicht MD. Key stages in mammary gland development: The cues that regulate ductal branching morphogenesis. Breast Cancer Research 8: 1, 2006 |
|
Fenton SE. Endocrine-Disrupting compounds and mammary gland development: Early exposure and later life consequences. Endocrinology S18, 2006 |
|
Raynaud A: Morphogenesis of the mammary gland. In Kon SK, Cowie AT (eds): Milk: The Mammary Gland and Its Secretion, p 3. Vol 1. New York: Academic Press, 1961 |
|
Neumann F, von Berswordt-Wallrabe R, Elger W et al: Aspects of androgen-dependent events as studied by anti-androgens. Recent Prog Horm Res 25: 337, 1970 |
|
Hamer DB: Gynaecomastia. Br J Surg 62: 326, 1975 |
|
Hartman CG: Case of supernumerary nipple in macacus rhesus, with remarks upon biology of polymastia and polythelia. J Mammal 8: 86, 1927 |
|
Kleinberg DL, Newman CB: The pituitary gland in primate mammary development: Evidence that prolactin is not essential. Ann N Y Acad Sci 446: 37, 1986 |
|
Kleinberg DL, Niemann W, Flamm E et al: Primary mammary development: Effects of hypophysectomy, prolactin inhibition, and growth hormone administration. J Clin Invest 75: 1943, 1985 |
|
Leung BS, Sasaki GH: Prolactin and progesterone effect on specific estradiol bindingin uterine and mammary tissue in vitro. Biochem Biophys Res Commun 55: 1180, 1973 |
|
Porter JC: Hormonal regulation of breast development and activity. J Invest Dermatol 63: 85, 1974 |
|
Sakakura T, Nishczuka Y, Dawe CJ: Mesenchyme dependent morphogenesis and epithelium-specific cytodifferentiation in mouse mammary gland. Science 194: 1439, 1976 |
|
Pasquino AM, Tebaldi L, Cioschi L et al: Premature thelarche: A follow-up study of 40 girls. Arch Dis Child 60: 1180, 1985 |
|
Ilicki A, Lewin RP, Kauli R et al: Premature thelarche: Natural history and sex hormone secretion in 68 girls. Acta Pediatr Scand 73: 756, 1984 |
|
Stanhope R, Abdulwahid NA, Adams J et al: Studies of gonadotropin pulsatility and pelvic ultrasound examination distinguish between isolated premature thelarche and central precocious puberty. Eur J Pediatr 145: 190, 1986 |
|
Pasguino AM, Pacrelle I, Passeri F et al: Progression of premature thelarche to central precocious puberty. J Pediatr 126: 11, 1995 |
|
Perel E, Davis S, Killinger DW: Androgen metabolism in male and female breast tissue. Steroids 37: 345, 1981 |
|
Rose DP: Hormones in breast fluid. Breast Cancer Res Treat 8: 25, 1986 |
|
Stadel BV, Webster LA, Rubin GL et al: Oral contraceptives and breast cancer in young women. Lancet 2: 970, 1985 |
|
Casey PM, Cerhan JR, Pruthi S. Oral contraceptive use and the risk of breast cancer. Mayo Clin Proc 83: 86, 2008 |
|
Depot medroxyprogesterone acetate and cancer: Memorandum from World Health Organization. Bull World Health Organ 64:375, 1986 |
|
Klaiber EL, Vogel W, Rako S. A critique of the Women’s Health Initiative hormone therapy study. Fert Steril 84: 1589, 2005 |
|
Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: Collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet 350: 1047, 1997 |
|
Colditz GA, Hankison SE, Hunter DJ et al: The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med 332: 1589, 1995 |
|
Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. JAMA 288: 221, 2002 |
|
The Women’s Health Initiative Steering Committee: Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial. JAMA 291: 1701, 2004 |
|
Farber JL (ed): Pathology, p 998. Philadelphia: JB Lippincott, 1988 |
|
Vorherr H: Fibrocystic breast disease: Pathophysiology, pathomorphology, clinical picture, and management. Am J Obstet Gynecol 154: 161, 1986 |
|
Page DL, Dupont WD, Rogers LW et al: Atypical hyperplastic lesions of the female breast. Cancer 50: 2698, 1985 |
|
Dogliotti L, Fioretti P, Melis GB et al: Current status of hormonal therapy of fibrocystic breast disease. Ann N Y Acad Sci 464: 360, 1986 |
|
Parlati E, Polinari U, Salvi G et al: A double-blind trial in the evaluation of bromocriptine in the treatment of breast disease. Ann N Y Acad Sci 464: 613, 1986 |
|
Winkler UH, Schindler AE, Brinkman US, et al. Cyclic progestin therapy for the management of mastopathy and mastodynia. Gynecol Endocrinol 6: 37, 2001 |
|
Shaaban MM, Morad F, Hassan A: Treatment of fibrocystic mastopathy by an antiestrogen, tamoxifen. Int J Gynecol Obstet 18: 348, 1980. |
|
Seltzer MH: The significance of breast complaints as correlated with age and breast cancer. Am Surg 58: 413, 1992 |
|
Pruthi S, Brandt K, Degnim A, et al. A multidisciplinary approach to the management of breast cancer, Part 1: Prevention and Diagnosis. Mayo Clin Proc 82: 999, 2007 |
|
American Cancer Society Guidelines for Breast Cancer Screening: Update 2003. CA Cancer J Clin 53: 141, 2003 |
|
Amir Q, Snow V, Sherif K, et al. Screening mammography for women 40 to 49 years of age: A clinical practice guideline from the American College of Physicians. Ann Intern Med 146: 511, 2007 |
|
American Cancer Society Guidelines for Breast Cancer Screening with MRI as an Adjunct to Mammography. CA Cancer J Clin 57: 75, 2007 |
|
Evans WP: Breast masses. Radiol Clin North Am 33: 1085, 1985 |